Winning Early, Treating Smart: A 2025 Playbook for IBD Clinicians
Six Data-Powered Moves to Elevate IBD Care and Improve Outcomes for Patients
IBD is hard for patients, and unmet clinical need remains substantial.
We must keep improving outcomes—both now and in the future. Today this means deploying our therapeutic toolkit effectively, using high-quality data to drive decision-making and always maintaining a patient centered approach.
Here are six data driven strategies to improve IBD outcomes in 2025:
1. Make a timely diagnosis of IBD
Diagnostic delay of IBD remains a big problem across the world.
The median diagnostic delay (time from first symptoms to diagnosis) was 8 and 4 months for Crohn’s disease and UC respectively. Of note, in one UK study almost 1 in 5 patients were waiting over five years for a diagnosis.
There are significantly worse clinical outcomes in patients with diagnostic delay.
In both Crohn’s disease and ulcerative colitis there is an increased risk of surgery with delayed diagnosis. A 2023 meta-analysis reported a four fold increased risk of colectomy in UC (OR 4.13) and almost double the risk of resectional surgery in CD (OR 1.88).
How to reduce the diagnostic delay?
Raise awareness across all healthcare providers and more broadly across society, continue the roll-out use of faecal calprotectin to primary care (and consumer direct to test), and tools like the Crohn’s Colitis UK Symptom Checker.
2. Treat effectively and (very) early in Crohn’s disease
The data are crystal clear - early effective therapy in Crohn’s disease modifies disease course and smashes through the therapeutic ceiling.
The data support IFX plus AZA started during the first month of diagnosis; so this is the approach for the moderate to high-risk patient, including all with a fistulising phenotype.
In moderate Crohn’s disease (the majority at presentation), extrapolation of Profile data to other effective therapies makes sense.
We use a lot of biosimilar adalimumab. It is highly effective, well tolerated and cheaper than oral 5-ASA! But there are trade-offs, notably with persistence, safety and sequencing. Now that USTE is biosimilar it also a first-line option for us, but currently 2-3x ADA prices.
My preference would be to use a p19 (risankizumab or guselkumab) for newly diagnosed patients with uncomplicated luminal Crohn’s disease, but this is not an option in the NHS.
After TNF failure, it is risankizumab or upadacitinib.
Outside of anti-TNF, I am not a fan of high unlicensed doses of advanced therapies.
Vedolizumab probably no impact with q4w (still an open debate - which in itself is extraordinary 10y on). Ustekinumab: Power study was inconclusive; recent GETAID study (presented at ECCO 2025) showed that iv re-induction plus q4w dosing were of minimal benefit.
Four weekly RISA? Not withstanding that I promised our lead pharmacist we would not do this, there is currently no data to support this (expensive) approach. And as for UPA - there is clearly a dose-toxicity relationship with all JAKi (all small molecules) and I am uncomfortable with maintenance doses of more than 30mg once daily.
Instead our preference is to use advanced combination therapy (ACT) - eg risankizumab q8w with upadacitinib (sometimes just for 12w induction at 45mg, and sometimes for maintenance when we try to get down to 15mg od).
Note that this all ACT is currently off-licence.
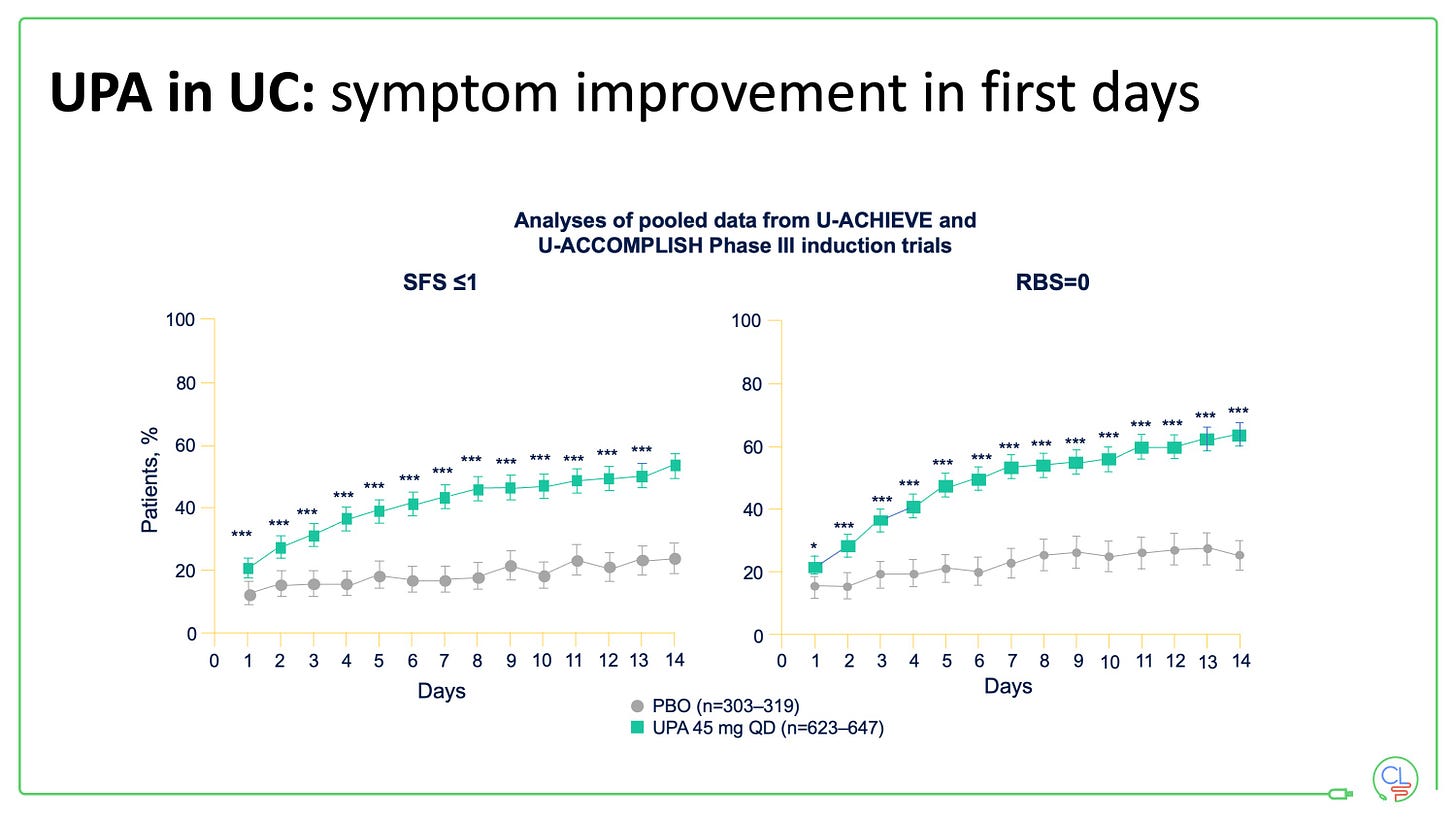
3. Get on top of flares quickly in ulcerative colitis
Do patients with UC need top-down therapy (like profile in CD)?
Short answer is no.
5-ASA is a fantastic treatment for mild-to-moderate UC. Optimise once daily oral dosing and add rectal therapy for induction (E2 and E3). Rectal 5-ASA therapy for proctitis (E1) is often all that is needed.
It is perfectly reasonable to use a course of steroids in UC. A one off course of MMX budesonide or oral prednisolone is fine. It may be all that a patient needs before continuing oral 5-ASA. BUT … know the rules to identify steroid resistance early (be prepared to escalate to AT after 2 weeks) and prevent patients from becoming steroid dependent (inexcusable in 2025).
Rapid flare management is critical in UC
You need robust and responsive systems of care to achieve this. We run a Monday to Friday flare clinic - one or two IBD patients identified from the IBD nurse helpline and triaged at the mid morning IBD huddle.
It works like this:
Identify flaring UC patient. Same day review at flare clinic - IBD nurse, consultant, pharmacist +./- dietitian. History, exam, bloods, stool sampling, pre-biologic screening, ultrasound, drug counselling.
And with JAK inhibitors - once daily tablets, same day collection from pharmacy, rapid onset of action, no need for steroids. We usually ask patients to wait 24 hours until the key bloods are back then start (depending on risk / benefit / prior lab availability).
Upadacitinib (UPA) is our go to for the flaring patient on the cusp of admission, filgotinib or etrasimod for many others, but we still use a lot of infliximab (mostly subcut) and vedolizumab. And we are eagerly anticipating more widespread availability of the p19’s in UC (plus head to head data with vedo).
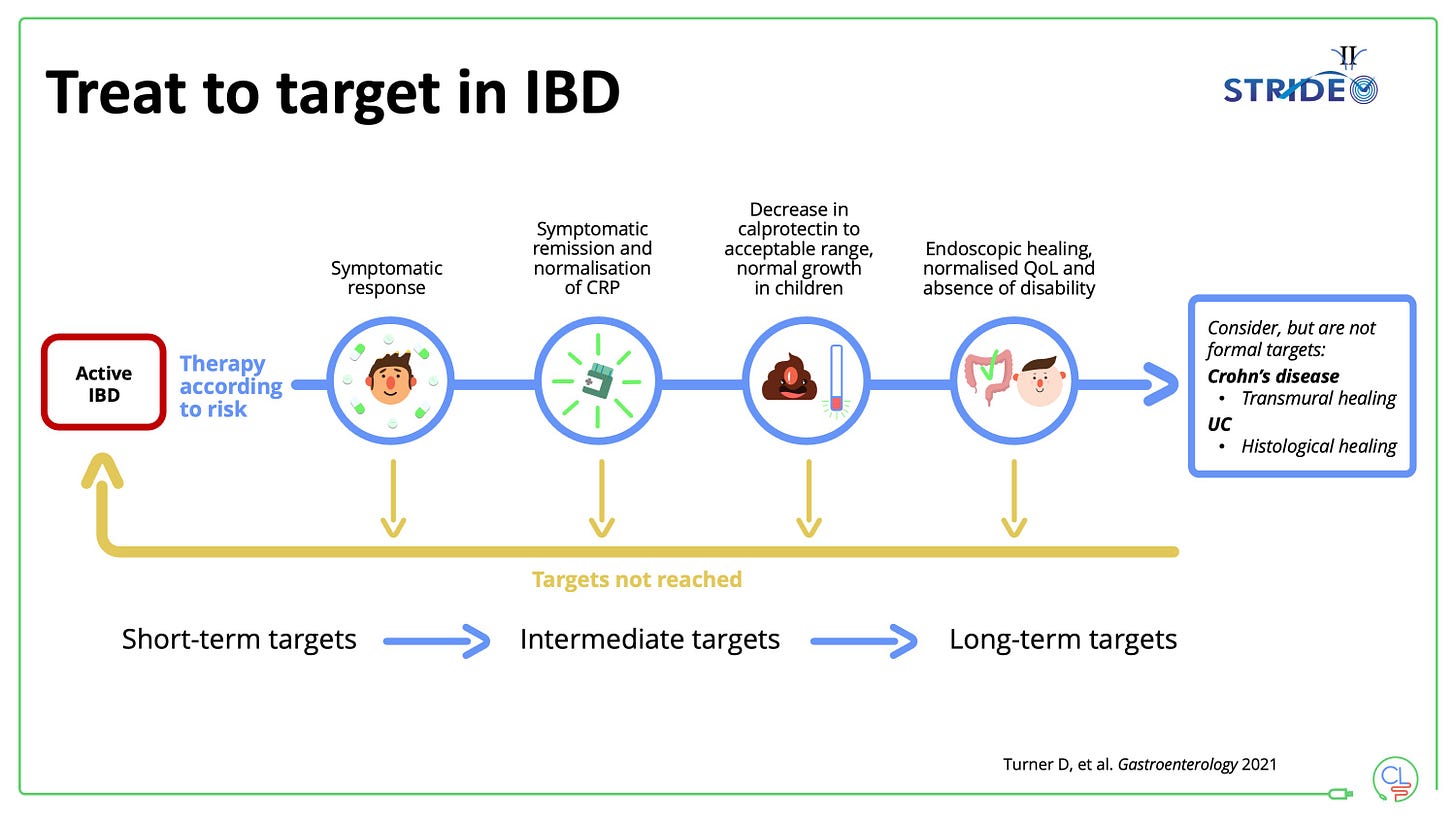
4. Use a treat-to-target strategy for the whole patient
The framework is simple, pragmatic and works.
It can be boiled down to: monitor, monitor, monitor and act on the results of monitoring.
As for much in life, if you can’t measure it, then you can’t improve it.
We use a system based on symptoms, PROs, bloods and calprotectin, intestinal ultrasound and colonoscopy.
We are developing a next generation state-of-the-art digital tool to transform IBD care: passive, frictionless monitoring is our goal.
5. Think carefully about drug positioning in 2025
Three simple rules to follow here:
think holistically with a patient-centred approach
don’t make a patient earn their right onto an advanced therapy
where possible use the best drug first
6. Keep an eye on the future … it is happening now
The next ten years of IBD therapeutics is going to be fast and furious. Here are a few big ticket items to look out for:
We will learn how to use current drugs better
For example, pulsing JAK inhibitors in both Crohn’s and UC (as we currently use steroids). I’d love to see innovative trial designs putting this to the test.
Combinations of existing advanced therapies
Advanced Combo Therapy (ACT) is already part of our practice for difficult to treat patients. Controlled trial data are eagerly awaited from RCTs including:
guselkumab (p19) plus golimumab (DUET-CD and DUET-UC)
vedolizumab plus upadacitinib in CD (VICTREVA)
vedolizumab plus tofacitinib in UC
risankizumab plus lutikizumab or ABBV-382 (anti-a4b7) platform trial in CD
New antibodies against existing targets
The company to watch here is Spyre Therapeutics who have made extended half-life antibodies (via a YTE modification in the Fc region to enable q3m or q6m subcut dosing) against all key IBD targets including IL23 (SPY003), a4b7 (SPY001) and TL1A (SPY002). They plan to test these alone and in combination over the coming years.
Completely novel targets
The list is long, but the near horizon involves:
At least three molecules in late phase testing that target TL1A; there are more in early phases.
Obifazimod (ABX464) up-regulating miR-124 and fine-tuning the immune response. I’m particularly excited to see how this molecules comes out after completing phase 3 testing in UC (soon) and CD.
Microbiome targets and dietary treatments
The potential for CRISPR gene editing and phage technology to “fix” the microbiome defect in IBD is huge.





Hi there,
I enjoyed your writing. I've spent the last 30 years as a gastroenterologist based in Cleveland, and for the past 16 years I've written a blog sharing insights into the medical profession. I just started a Substack to share my thoughts and advice. My latest post is about chronic abdominal pain. I'm hoping it may prove relevant to you, and that you'll considering following along.
https://mkirsch.substack.com/p/whats-the-cause-of-chronic-abdominal
Thanks!