If all we do for the next ten years is implement what we know works well – for everyone, everywhere - we will totally transform disease outcomes
Before we get started please take a moment to complete this survey for the Lancet Gastroenterology Commission on Global IBD. You will find the survey here. The survey is for healthcare professionals looking after patients with IBD anywhere in the world. The estimated completion time is approximately 20 minutes.
I started working in IBD 22 years ago.
Outcomes for many IBD patients were awful – chronic continuous symptoms, frequent hospitalisations, major surgeries, and all too often gut failure necessitating intravenous feeding. Not to mention the profound psychological burden – which we barely asked about in those days.
And looking back, none of this was particularly surprising. We had no meaningful treatment targets - the best we could do was “steroid free clinical remission”. It's just as well our targets weren't stricter back then, since we lacked therapies capable of achieving mucosal healing.
We marinated patients in steroids and did the best we could to navigate the toxicity of conventional immunosuppressants – maximising doses in the vain hope of eking out as much efficacy as possible.
None of this was necessarily bad management – we just didn’t have the right tools for the job.
But wasn’t it 20 years ago that the biologicals revolution started?
Yes! BUT … in those early days we had no idea how to use the new drugs properly. We made patients “earn their right” onto an anti-TNF, waiting until their disease was dominated by irreversible fibrosis and devastating penetrating complications – the natural sequelae of years of uncontrolled inflammation.
(And that was just in Crohn’s disease – for most, these drugs were not available in ulcerative colitis until 2014.)
And then? We used infliximab episodically, as monotherapy, and were surprised when patients lost response.
To be fair, we didn’t realise then how immunogenic the first generation monoclonal antibodies were: infliximab was the worst culprit with its 25% murine component, and even the “fully humanised” adalimumab was not quite the full solution we thought.
We figured this out … combination therapy with an immunosuppressant.
For about 2 years, until the hepato-splenic T cell lymphoma story derailed combo therapy for what seemed like an eternity. We were so fixed on the rarest of combinations we lost sight of the big picture –
It isn’t the drugs that are dangerous, it is the active disease.
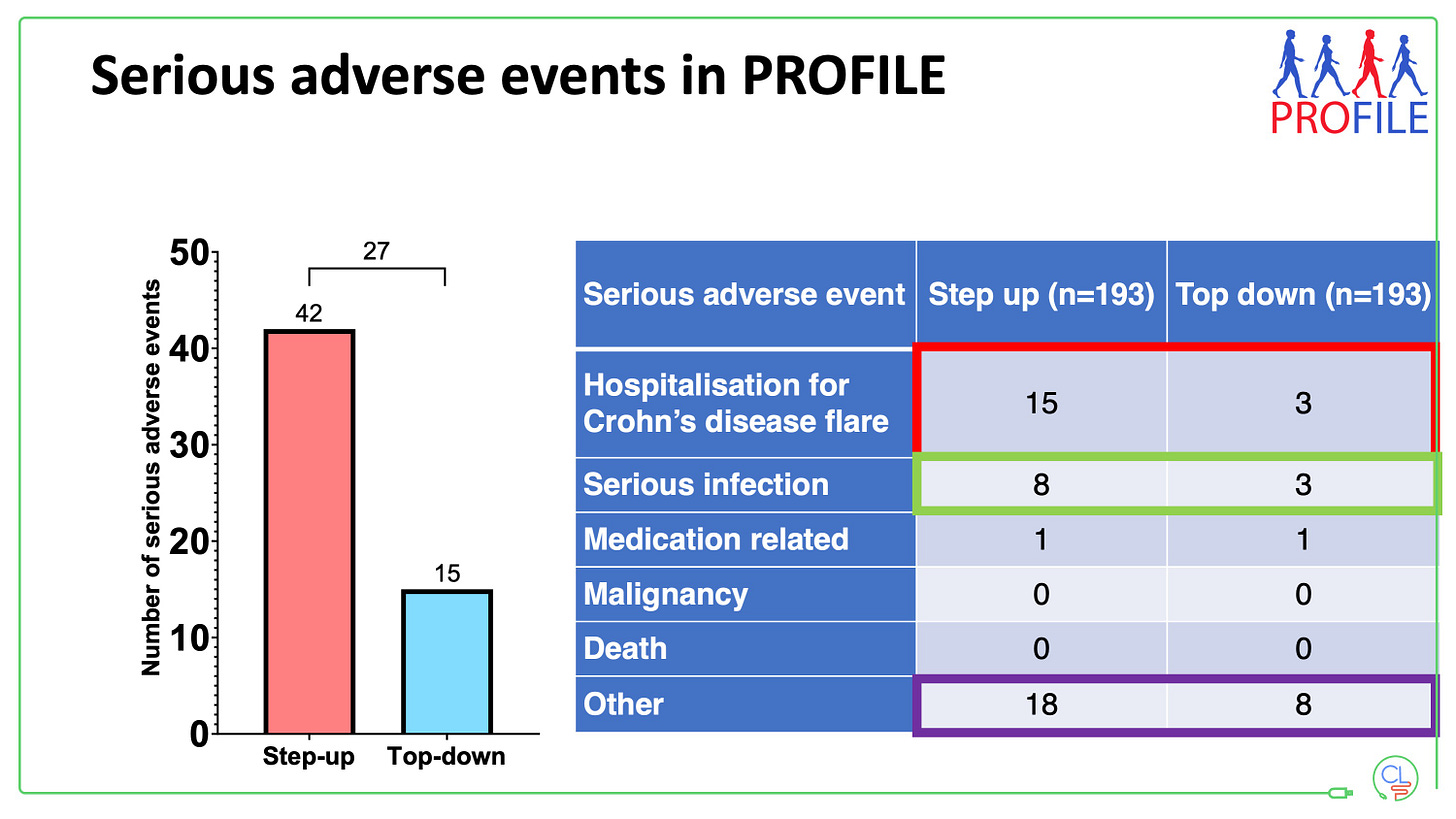
This was shown most recently by PROFILE – half the number of adverse events with combo IFX plus AZA versus step up therapy; less than half the number of serious AEs, including serious infections. And two pivotal meta-analyses on the harms of placebo in IBD drug trials: More dangerous adverse events (including death) than with any of the drugs in phIII trials.
And this brings me to my main point.
Over the last 20 years we have conducted studies, analysed data, iterated practice and learned. At least ten landmark post-registration clinical studies just on anti-TNF therapy in IBD.
And we have caught the attention of big pharma, brilliant academics, and policy makers, and the IBD community has expanded enormously. IBD has climbed the agenda, been prioritised, and we ARE SEEING THE REWARDS.
New data, new drugs, new target driven treatment principles. That have dramatically improved outcomes
Treat early
Use effective therapy (don’t worry too much about which one)
Don’t make patients earn their right onto advanced therapies
Don’t save your best therapy until the end
Use a holistic, patient-centered approach
Use target driven management (or, in other words: monitor, monitor, monitor and act on the results of the monitoring)
Two decades of relentless forward progress.
Do we have all the IBD management tools we need today?
No. Too many patients will fail to respond adequately to any available therapy. Our therapies are often still too expensive and not available to all. Our monitoring tools are invasive or unpleasant for patients.
Plus, the unrelenting rise in IBD across the world presents a uniquely urgent problem.
Yet … if all we do today is implement what we know works well – for everyone, everywhere – we will transform outcomes for IBD patients.
And while we do that, we will continue to learn, new drugs will come to market, and the patents covering existing drugs will expire.
I’ll leave you with this final thought: By 2032, every currently approved drug for IBD will be available as biosimilar or generic versions.
And that will be a game changer.







Hi there,
I've spent the last 30 years as a gastroenterologist based in Cleveland, and for the past 16 years I've written a blog sharing insights into the medical profession. I just started a Substack to share my thoughts and advice. My latest post is about chronic abdominal pain. I'm hoping it may prove relevant to you, and that you'll considering following along.
https://mkirsch.substack.com/p/whats-the-cause-of-chronic-abdominal
Thanks!
Hola, muy buena reflexión, Para la Salud pública, en ámbito hospitalario donde me desempeño, aún estamos como su inicio, hace 20 años. Saludos, es muy esperanzador leer sus notas, espero que el futuro llegue pronto a nuestra realidad sanitaria