I started working in IBD in 2003.
This was at the very start of the biologic era. Infliximab was new and expensive and typically used late in the disease course when everything else had failed. We used the drug episodically, often as monotherapy and whilst it was transformative for so many, for others the irreversible bowel damage was not salvageable with this new drug. We were giving our most effective anti-inflammation therapy late in the disease course. We had too many of our patients marinated in steroids and stuck on conventional immunomodulators that weren’t working.
We got things wrong but not out of bad faith, just lack of experience and data. And we have learned fast.
We have come a long way in the last 20 years
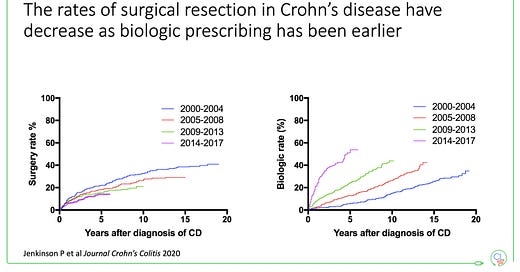
You can see this in the patterns of biologic use and surgical rates in Crohn’s disease in Edinburgh since then. Early biologic use associated with fewer surgeries.
With 2 decades of controlled clinical trial data, real-world evidence and the advent of biosimilar anti-TNF we now have the tools to improve outcomes for many people with IBD, when the conditions are correct (e.g. newly diagnosed Crohn’s disease).
The multi-drug era of IBD is firmly here
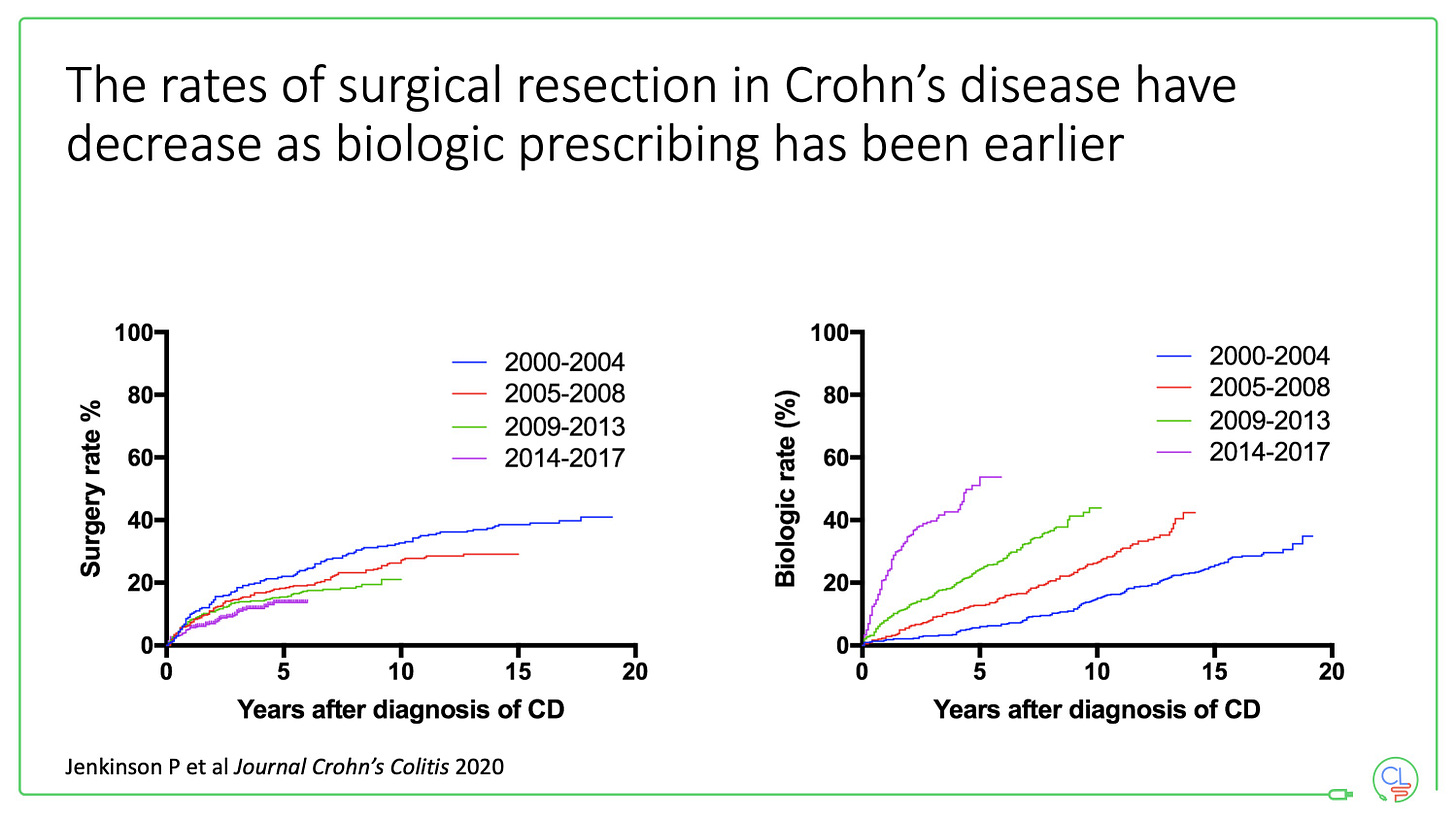
In the last 7 years we have added first vedolizumab, then ustekinumab and most recently, tofacitinib to our therapeutic line-up. Five different classes of effective drug - anti-TNF, anti-integrin, anti-IL12/23 and JAK inhibitor - equals the multi-drug era.
Here you can see the timeline of new therapies up until the present day. The most recent additions have been subcutaneous infliximab and vedolizumab. Flexibility of mode of delivery has never been greater - this choice is good for healthcare practitioners and patients, especially during the pandemic.
Let's now turn to what comes next …
The next few years look set to be non-stop in the world of IBD therapeutics. This is great news for us as healthcare practitioners - we have a lot of patients whose need is not met by current therapies, and for our patients - current and future.
In the next 3 years, I estimate that 7 new molecules will come to the clinic (across UC and Crohn’s disease combined). You can read the summary of these here:


I’ve pointed out above where these studies have completed their registration trials.
A brief summary: ozanimod (UC), filgotinib (UC), rizankizumab (CD), upadacitinib (UC) and mirikizumab (UC) have all completed for at least one indication. The data have been presented - ECCO and UEG meetings this year were packed full of new data - but most await formal full publication in peer reviewed journals.
You can see the near future therapeutic landscape is heavily biased to UC. Arguable the much greater and more urgent clinical need is in Crohn’s disease.
Etralisamod and gulsekumab phase 3 programmes are on-going - the latter with an extensive suite of placebo controlled, head-to-head and combination studies.
Filgotinib, upadacinitinib and mirikizumab are all also being tested in Crohn’s disease. Rizankizumab is being tested in UC.
Now let’s talk about Etrolizumab
One of the molecules that didn’t make my shortlist of 7 was etrolizumab.
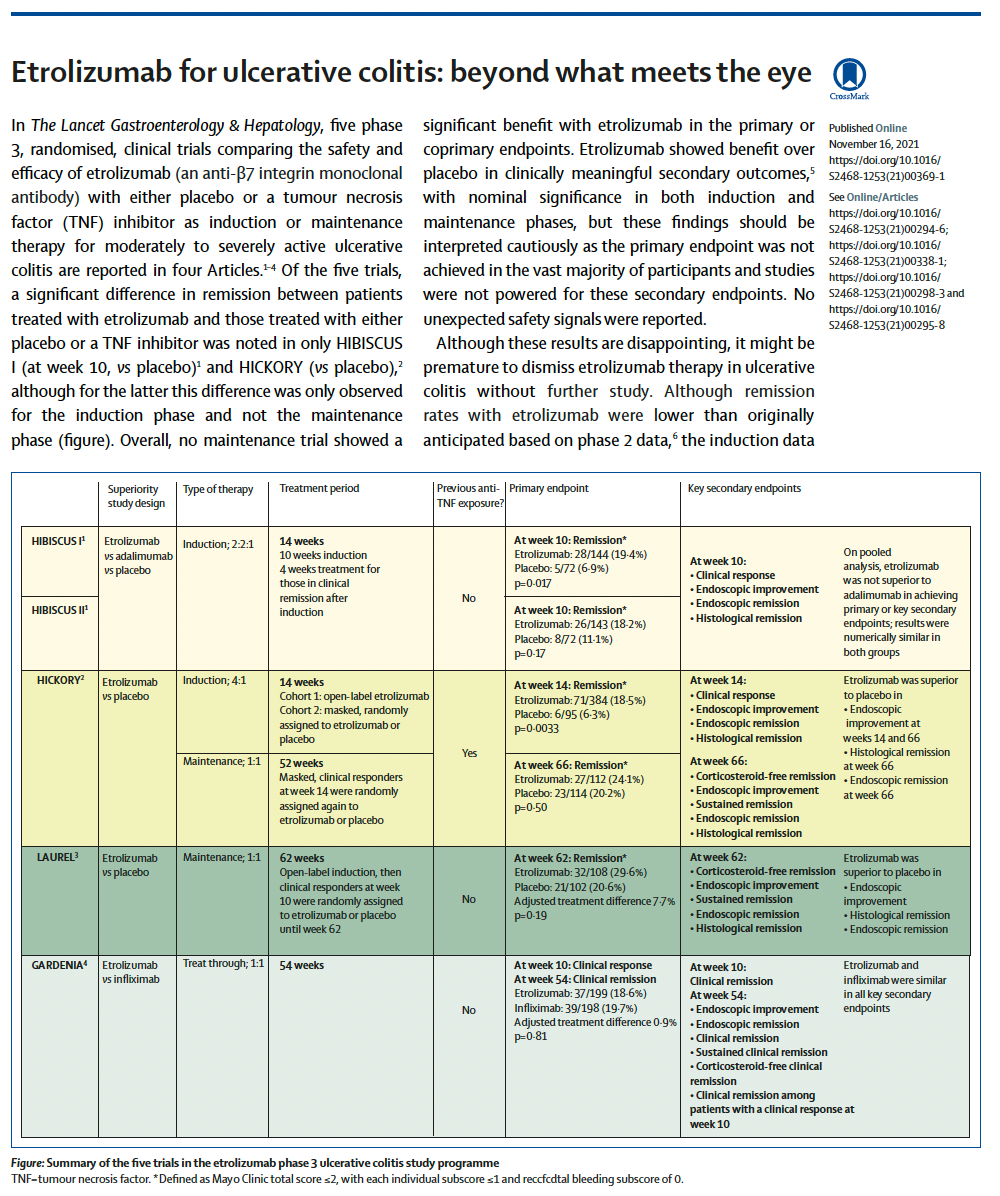
A year ago at the virtual UEG meeting (October 2020), a series of phase 3 randomised clinical trials reported their primary and key secondary endpoints. All FIVE of the RCTs have today reported in full in the journal Lancet Gastroenterology and Hepatology. This was a very ambitious ph 3 programme that included induction and maintenance studies against placebo, and two head-to-head studies: one against adalimumab and one against infliximab.
The results are disappointing. None of the studies showed evidence of benefit at the end of maintenance against placebo and there was no evidence of superiority against either anti-TNF agent.
Manasi Agrawal and Bram Verstock have written a really excellent commentary to accompany the publication of these data in the same journal.
To summarise the key point that they raise:
the induction effect was consistent across studies at about 20%
was the effect size assumption too high? (based on the ph2 data)
the placebo response was quite high
the dosing was perhaps too low
encouraging signals of efficacy against adalimumab (exploratory endpoint) and infliximab (primary endpoint)
Was this over-ambitious as a phase 3 study programme? I would agree that it probably was - you want to make sure you hit your primary endpoints. However, I also agree that we should admire this ambition. We want to see more head to head studies and fewer placebo controlled studies in IBD.
Interesting side note: all the phase 3 studies are named after some kind of shrub - hibiscus - hickory - laurel - gardenia.
Etrolizumab development continues in earnest for Crohn’s disease.
Back to some good news for HCPs and patients
The biosimilar revolution is continuing. Within the next 5 years we will have access to biosimilar ustekinumab and vedolizumab and generic tofacitinib.


Plus we are finally making significantly progress understanding the role of diet and the microbiome in IBD - cause and consequences. Both dietary and microbial therapies are in development. This will take time. They may be adjuncts to drug therapy for many. But they may well help us smash through the therapeutic ceiling in IBD.
So what am I most excited for in the next 5 years?
Here are the things I am most excited for with new therapies for IBD:
Showing the on-going benefit of early effective therapy in Crohn’s disease
Preventing hospitalisations for acute severe colitis by expediently treating flares with rapidly acting agents
Having choice for patients across different mechanisms of action and modes of delivery; systemically acting versus gut-specific; oral versus iv versus subcut;
Drugs with high levels of efficacy, rapid onset of action and excellent safety
Drugs that work when anti-TNF has failed
Combination of therapies made possible where companies have multiple assets (e.g. ustekinumab and golimumab; rizankizumab and adalimumab) and with multiple biosimilar MoA’s
Effective dietary strategies and microbial thearpies
Precision medicine that works
With this new dawn of IBD therapeutics approaching we have a new set of challenges as a community to rise to - including drug positioning and ensuring equitable access to affordable effective therapies across the world.
To do that we will need to develop deep community across healthcare professionals, academics, the pharmaceutical industry, biotech, funders, payers, and most importantly patients.


Very nice review - thanks, Charlie, and look forward to future posts!
Thanks for your review Charlie. Are you aware of any DNA studies targetting Crohn's or Colitis? Could this be an option in the future for patients who do not respond well to medications? And could it be more cost effective?