Choice of first biologic in Crohn's disease - key takeaways from the SEAVUE trial
Highlighting the importance of early effective therapy
Thank you for reading Atomic IBD. Welcome to all the new subscribers. If you have not yet subscribed please do so now to receive direct in your inbox.
For comments, feedback and ideas please email interesting@atomicibd.com
What do we learn from the SEAVUE trial?
Adalimumab and ustekinumab are both equally effective in early Crohn's disease.
That is the key takeaway from the SEAVUE trial published in full in The Lancet today. In this randomised, double-blind, parallel-group, active-comparator, phase 3b trial patients with bionaive moderately active Crohn’s disease were randomised to adalimumab or ustekinumab monotherapy.
Important things to note about the SEAVUE trial design:
patients had a relatively short duration of Crohn’s disease (median 2.6y; equivalent to that seen in the CALM study)
active inflammation and symptoms (CDAI 220-450) was needed at baseline, but this could have been only one ulcer at endoscopy
patients had to discontinue immunosuppressants (AZA/MP/Mtx) at least 3 weeks before randomisation
the double dummy design was somewhat imperfect - boxing was the same but the ADA and USTE syringes were different
no dose escalation was allowed - ADA maintenance was 40mg every other week; USTE was 90mg every 8 weeks
There were no differences between ADA and USTE across all key primary and secondary efficacy endpoints
The primary endpoint was clinical remission at week 52 - reached in 65% and 61% of patients on USTE and ADA respectively. These are impressive remission rates at one year, especially noting only a minimal reduction to steroid-free remission.
The rates of clinical remission at various timepoints show a very striking similarity between the two drugs. There may be an early signal of waning efficacy with adalimuamb from 6 - 12 months (which would be anticipated from the PK data), but this is at best speculative given the small delta.
Again both drugs are working quickly and effectively in this early bionaive Crohn’s disease cohort.
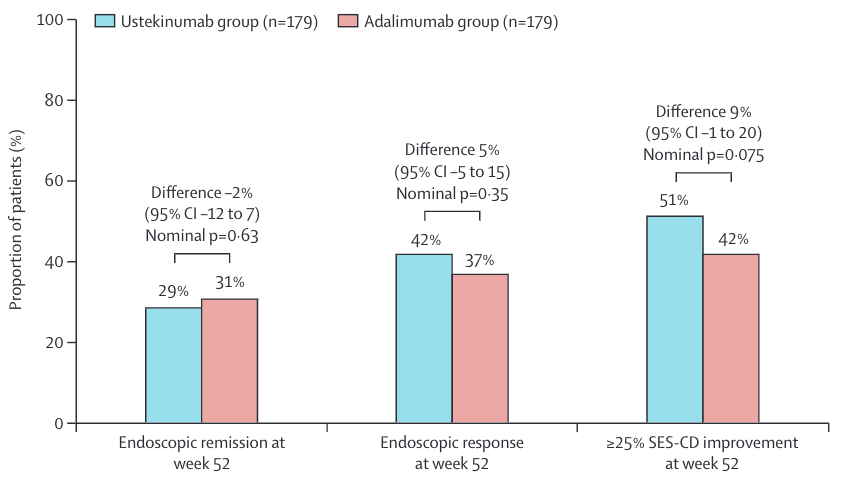
The endoscopy data from SEAVUE provide a very useful comparison of mucosal healing rates at 1 year of therapy with either biologic.
Again there was no difference between the drugs:
There are a number of other key points from the full paper:
A high rate of anti-drug antibodies to adalimumab, but this did not correlate with efficacy up to 52 weeks
Fewer patients on ADA completed 52 weeks of treatment (149/195) compared with USTE (162/191)
There was no significant phenotypic sub-groups who responded better to USTE versus ADA; this included disease duration and ileal versus colonic disease
The most important limitations to SEAVUE are:
the lack of dose optimisation
the primary endpoint of 52 weeks
Randomised IBD trials with readouts at 2 years and longer will be deeply informative.
What are the implications for clinical practice today?
Both ADA and USTE are highly effective in early Crohn’s disease.
This is using both drugs as monotherapy agents without dose escalation and as first line biologics. Dose escalation and combination therapy would be expected to give additional benefit to ADA treated patients. Long-term persistence is expected to be superior with USTE treatment given very low rates of immunogenicity. Long-term safety likely also favours USTE.
I would happily prescribe either drug as first line biologic in Crohn’s disease.
However, we live in a biosimilar world where a year of adalimumab therapy is the same price as a year of optimised 5ASA therapy.
The cost arguments strongly favour adalimumab first-line therapy for Crohn’s disease.
A simple set of recommendations might therefore be:
treat Crohn’s disease early with an effective agent = anti-TNF or USTE but not thiopurine monotherapy
complex (esp fistulising patients) prefer anti-TNF combination therapy
moderately active patients start adalimumab monotherapy
monitor TDM and dose optimise / add thiopurine as needed
if you do not start a biologic at diagnosis monitor CRP and calprotectin every 1-2m
IF CRP and calprotectin not falling below 5mg/L and 250mcg/g respectively then start biologic therapy
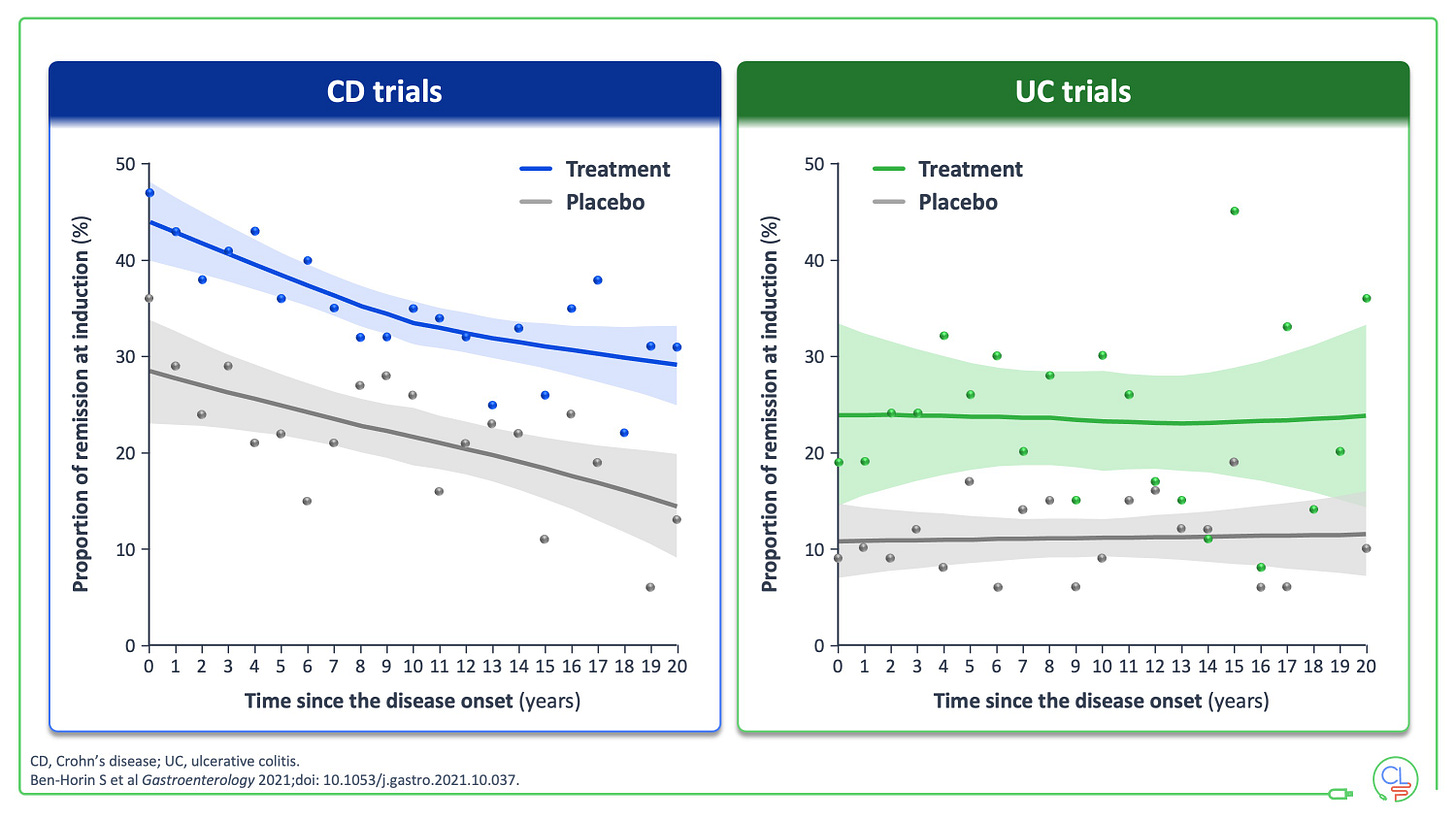
Overwhelming data now support early effective therapy in Crohn’s disease.
This was clearly demonstrated in a beautiful individual patient level meta-analysis of phase 3 trial data by Shomron Ben-Horin, published in Gastroenterology. This showed that shorter disease duration resulted in higher clinical remission following induction in Crohn’s disease.
This effect is not seen in UC where expedient management of flares is key.
A few thoughts on drug sequencing
Patients who fail and anti-TNF are harder to treat with USTE and VEDO. We see this in the clinic with Crohn’s disease patients where we frequently need to dose escalate to 4 weekly USTE (30% at a year in our cohort).
The drug pipeline is starting to offer some hope for a way around this with monoclonal antibodies targeting p19 and JAK1 inhibitors showing promising efficacy in anti-TNF experienced patients.
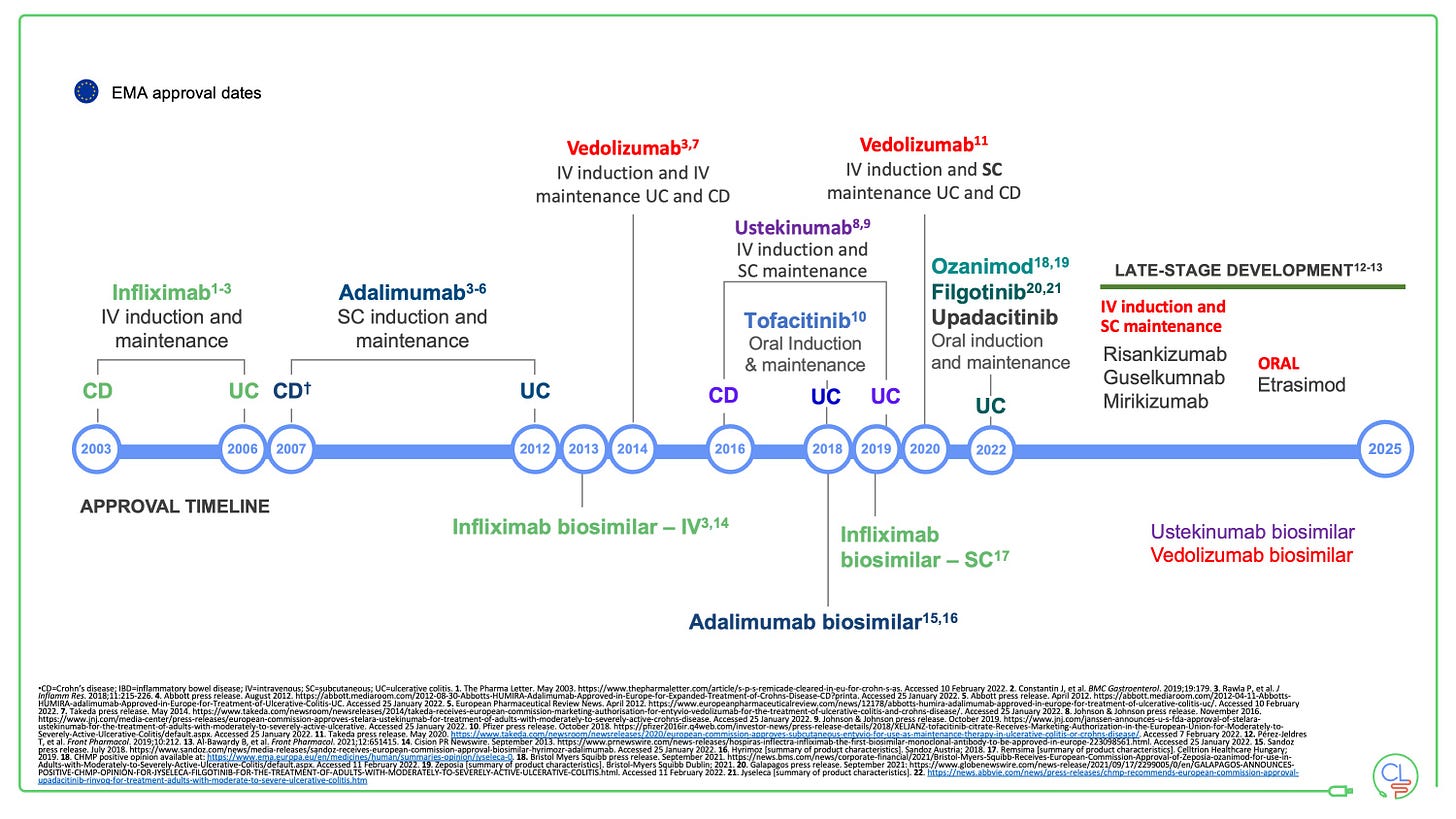
Usetekinumab will be available as a biosimilar soon
The USTE patent expiry is September 2023 in the USA and early 2024 in Europe.
We expect USTE biosimilars from Celltrion (CT-P43) and Amgen (ABP654) to be ready to launch then. Once more the expectation is that biosimilars will significantly alter the treatment landscape in IBD.
The SEAVUE data are incredibly informative now, and will be even more so then.