After TNF failure in Crohn's disease: What is the optimal treatment strategy?
Drug sequencing in Crohn's disease and the data from SEQUENCE
Infliximab and adalimumab remain the most widely prescribed first line biologics in Crohn’s disease, despite the arrival of newer therapies.
They work quickly, in the majority of patients, with good rates of mucosal healing and an acceptable safety profile.
However, there are three major problems with anti-TNFs:
They don’t work for everyone (primary non-response of approx 30%)
They don’t work forever (immunogenicity drives secondary loss of response)
After TNF failure, other drugs work less well.
Sequencing of therapies in Crohn’s disease has been troublesome for some time due to this third problem.
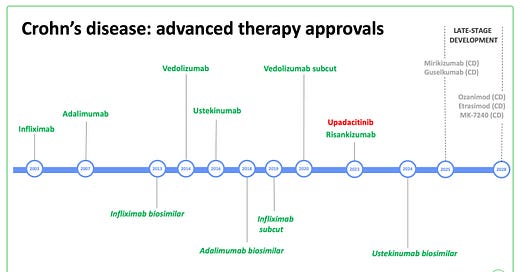
Recent historical perspectives
Ten years ago, patients failing their second anti-TNF (usually adalimumab) were stuck on 40mg once weekly with nowhere to go.
In 2014, vedolizumab arrived on the scene and was, by default, our go to drug after TNF failure.
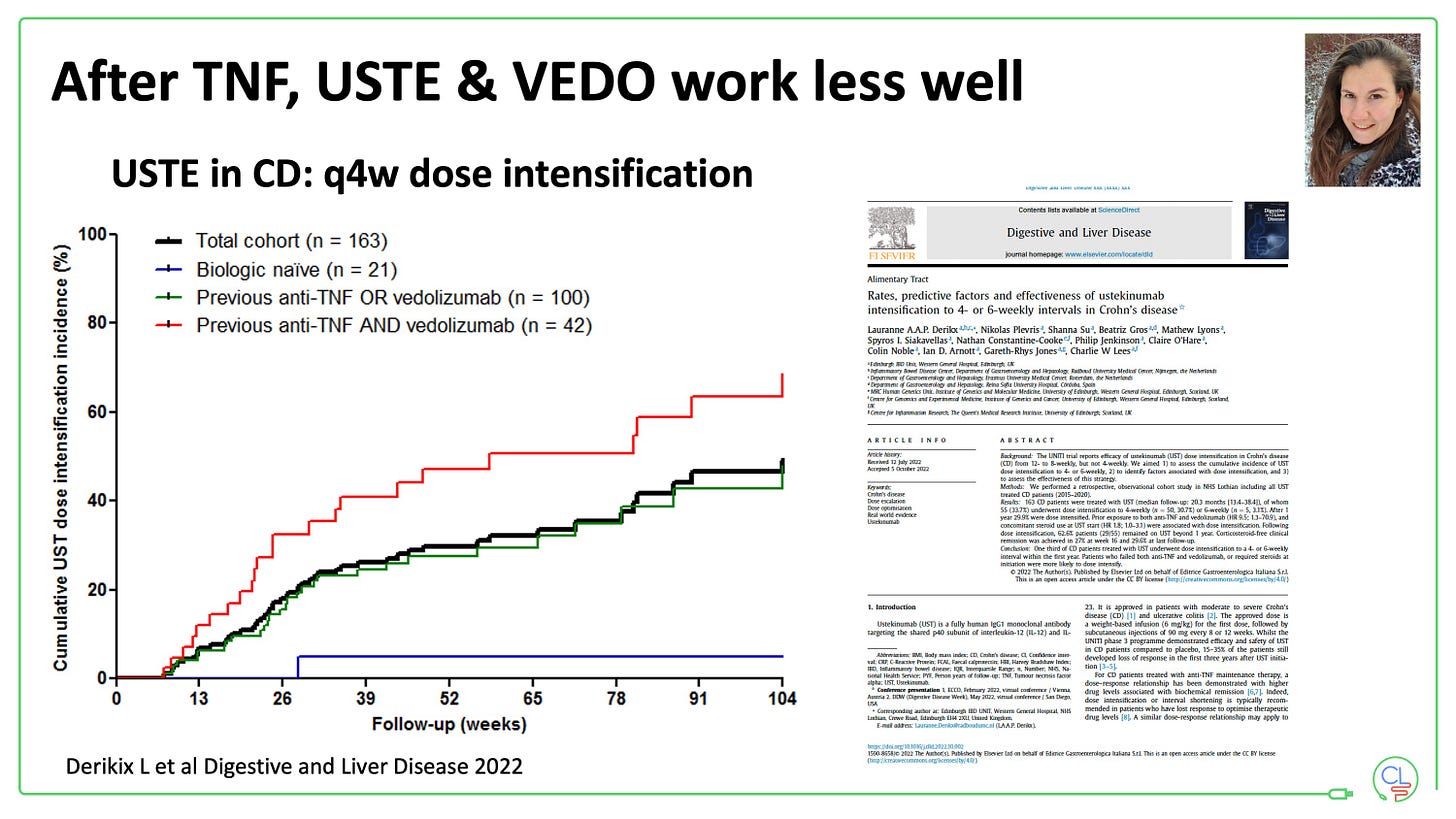
By 2016, ustekinumab came with much promise as another option in this situation.
Early experience, backed up by multiple real-world retrospective datasets, suggested ustekinumab was performing better than vedolizuamb after TNF failure.
But the reality is that neither works very well in this setting.
Both drugs work well as first line agents; SEAVUE being the key data point for ustekinumab. But we now live in a biosimilar world, where any cost-effectiveness analysis means an anti-TNF first strategy for the vast majority (N America and Australia are the exceptions here).
Note the same arguments don’t apply in ulcerative colitis.
And so up until very recently - our Crohn’s disease sequence has gone
First anti-TNF (mono or combo)
Second anti-TNF combo (after secondary LoR to first)
Ustekinumab with off-licence escalation to q4w in 30%
Vedolizumab
New molecules challenge the status quo in Crohn’s disease
In 2023, upadacitinib and risankizumab arrived on the scene for Crohn’s disease.
These two new therapies are changing the game after TNF failure in Crohn’s disease.
Upadacitinib - a small molecule JAK1 inhibitor, a once daily tablet
Risankizumab - a biologic targeting p19, and thus specifically blocking IL-23
Early experience with upadacinitib
We have been using upadacitinib for Crohn’s disease locally over the past 12 months.
Initially in multi-drug refractory patients - many who had failed infliximab, adalimumab, ustekinumab, vedolizumab (and others) - we were impressed by the rapid and complete response in a significant proportion.
Many of these patients had been ‘stuck’ for some time with no clear escape route (not suitable for surgery and not eligible for clinical trials).
We are now positioning upadacinitib earlier in the treatment sequence - either after ustekinumab or directly after TNF failure.
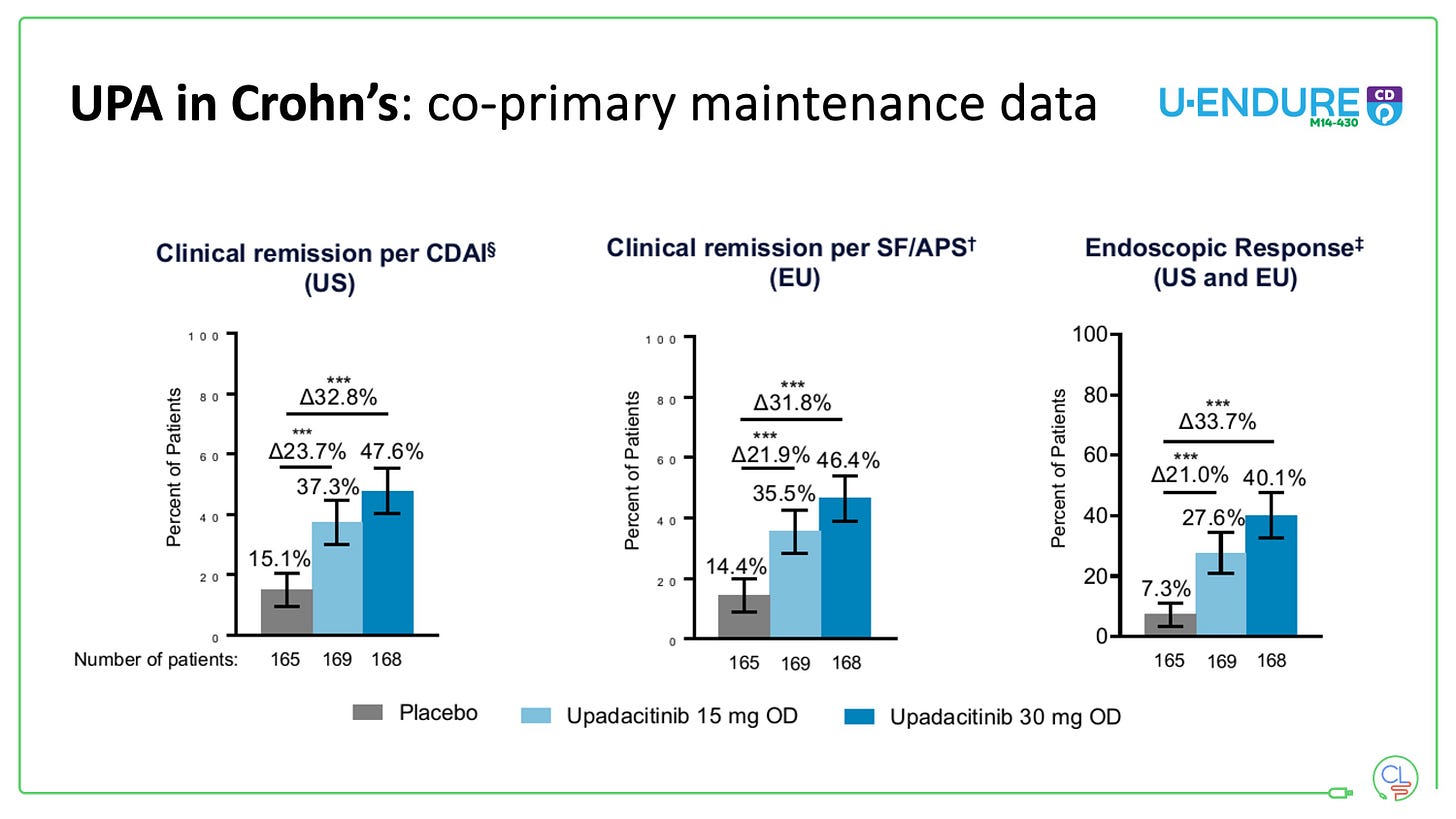
Our experience in our first 70 patients who have completed at least 12 weeks of induction, demonstrates high levels of effectiveness, with a need for the 30mg maintenance dose for the majority (we step down to this post-induction as per routine).
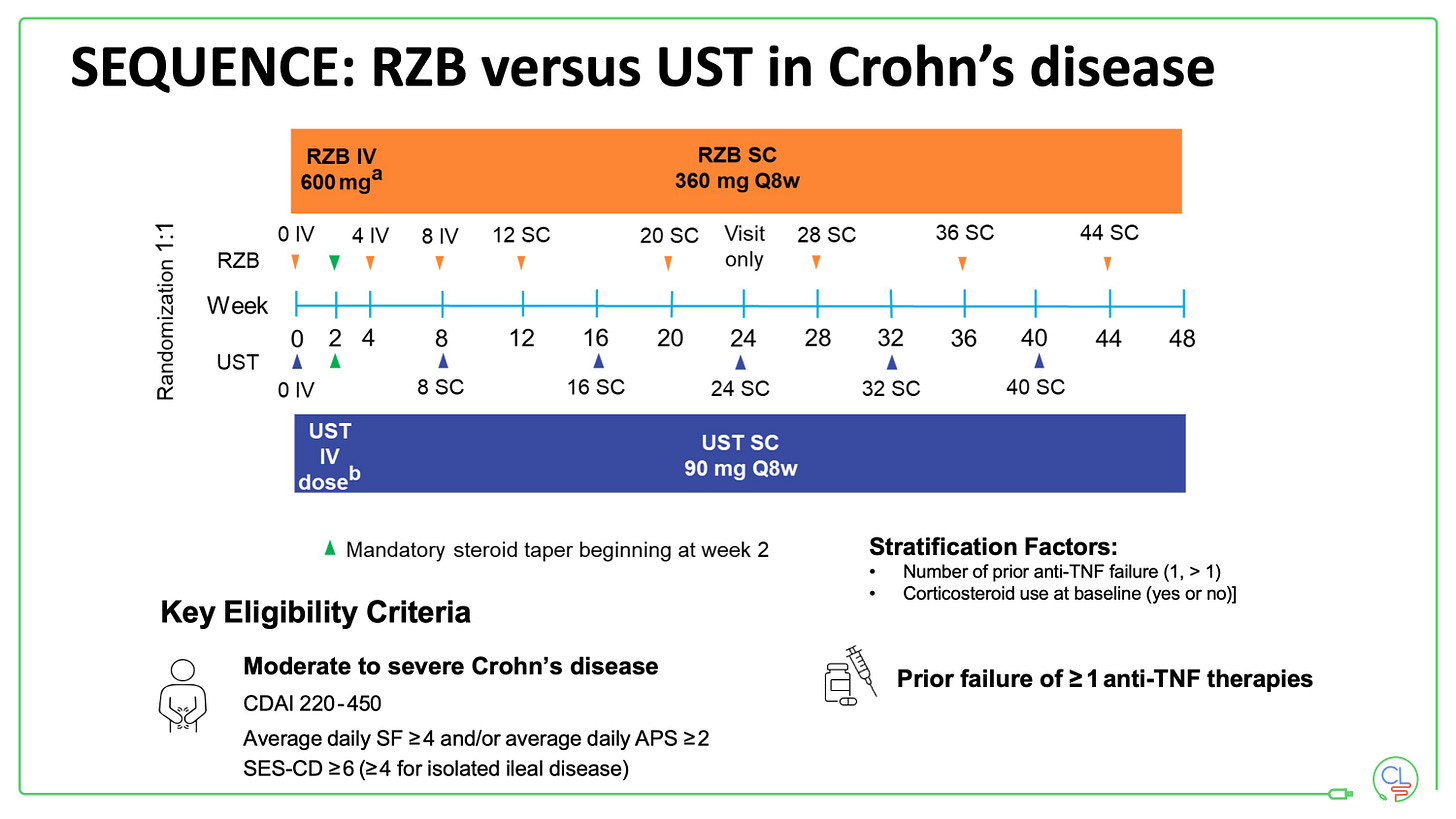
Risankizumab after TNF failure - SEQUENCE
Risankizumab has become available more recently and made big waves at UEG Week this year with first presentation of the SEQUENCE data.
We had used the drug 12-18 months ago in 24 patients through the early access scheme. 11 of these highly drug refractory patients remain in remission on the drug to date.
Now equipped with a new on-body device for the maintenance dose and a full suite of approvals we are able to prescribe the drug again.
Positioning will largely be after TNF which is why the SEQUENCE data are so important.
Patients in SEQUENCE had moderate to severely active Crohn’s disease and had failed at least one anti-TNF. There were randomised to risankizumab (RZB) or ustekinumab (UST) as per the schema.
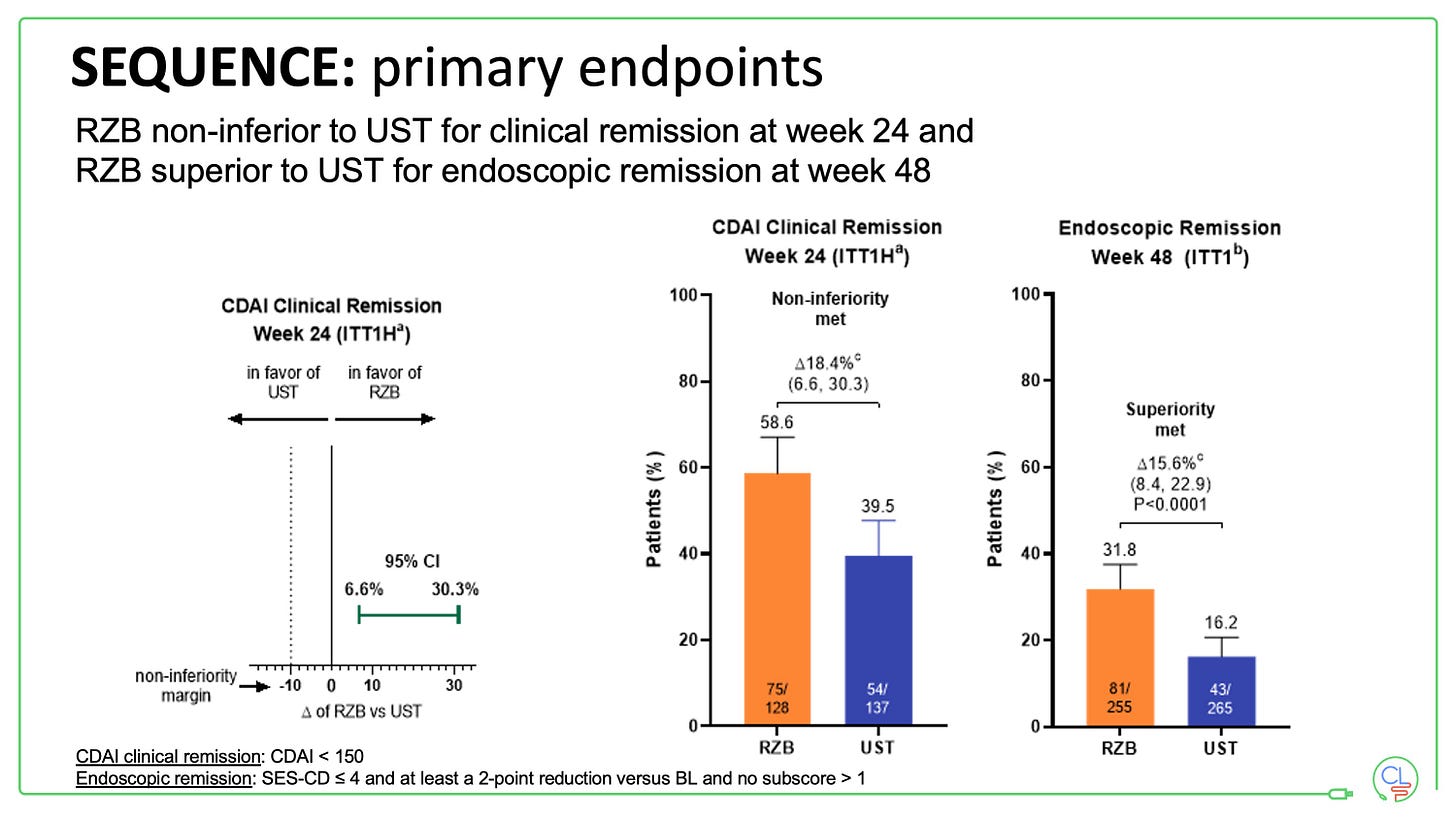
Both primary endpoints in the study were met:
RZB was non-inferior to UST for clinical remission at week 24 (in fact it demonstrates superiority for this endpoint)
RZB was superior to UST for endoscopic remission at week 48
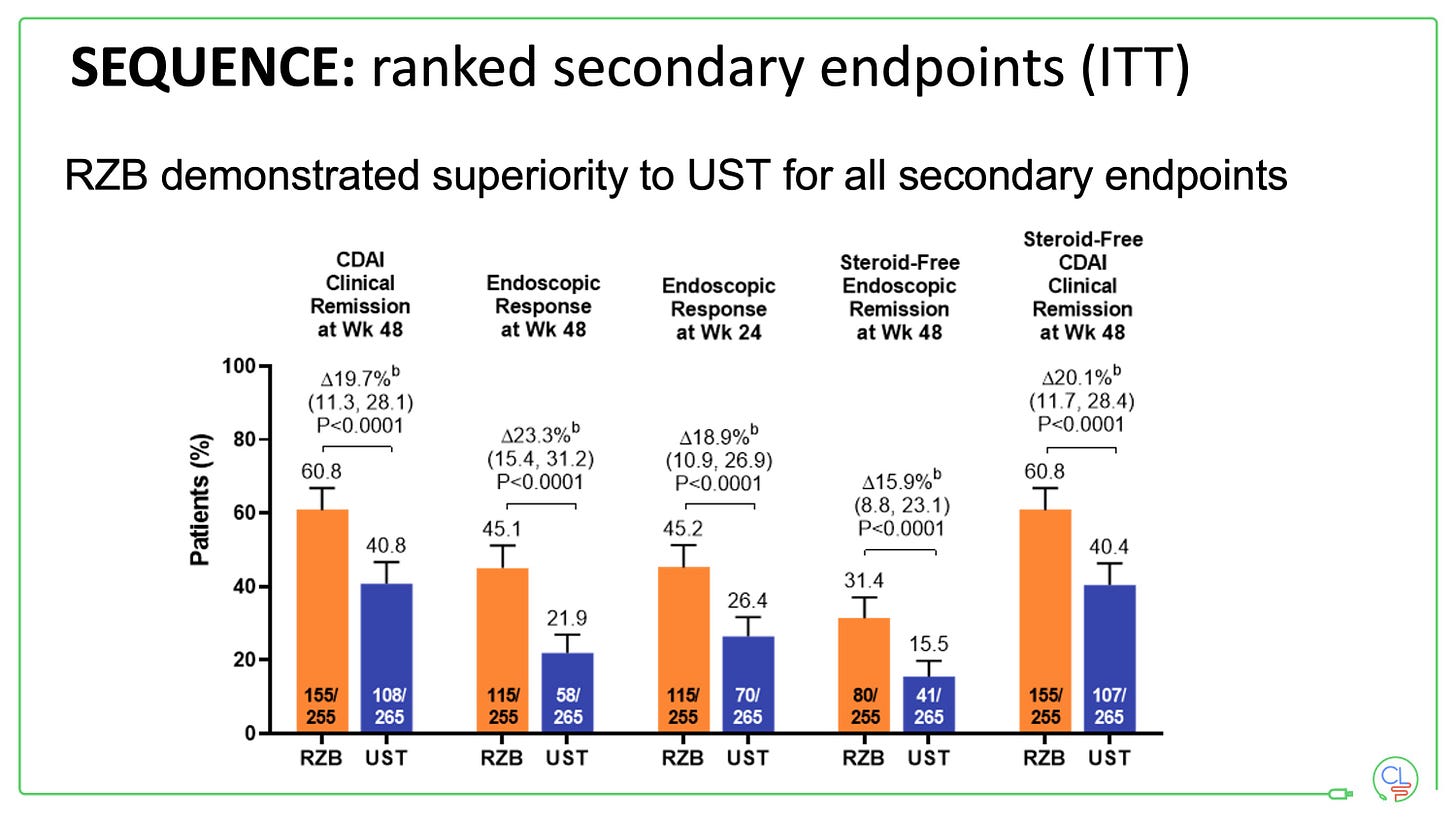
All key secondary endpoints were also met.
When interpreting these data it is important to bear in mind that SEQUENCE was not fully blinded. Investigators were blinded to treatment assignment at the point of all assessments, but patients were not.
Does this matter?
The authors state that it does not, that centrally read and blinded endoscopy can not be influenced by the lack of patient blinding.
But I’m not so sure.
Patients were aware of what treatment arm they were in. They elected to take part in a clinical trial of an exciting new therapy for Crohn’s disease. If you know you are getting the new “better” drug you might be pretty excited, and inclined to modify behaviour - eat better, exercise more, decrease stress, sleep better, comply with all other aspects of your care just that little bit better. This holistic focus might in turn help improve your Crohn’s disease.
Would this account for all the impressive delta seen for endoscopic remission? No I don’t think so, but maybe for at least some of it.
What about mirikizumab and guselkumab?
Most recently we heard - only in press release format - the first results from VIVID-1.
Mirikizumab versus placebo versus ustekinumab in Crohn’s disease - fully blinded pivotal phase 3 trial.
Mirizumab demonstrated impressive superiority to placebo - thus satisfying key efficacy endpoints.
Against ustekinumab, mirikizumab met non-inferiority to ustekinumab for clinical remission at week 24, but did not meet significance threshold for superiority of endoscopic remission at week 48.
We eagerly await the full VIVID-1 dataset and the results from phase 3 GALAXI study of guselkumab (expected at DDW 2024).
Further down the line we have the exciting prospect of data from DUET-CD - a combination therapy trial of guselkumab and golimumab.
One last consideration - biosimilar ustekinumab
The data and early experience support moving to upadacitinib or risankizumab after TNF failure.
Both appear superior to ustekinuamb in this setting.
BUT, in the second half of 2024 we will be in an ustekinumab biosimilar world (Biosimilars line up for launch).
QUESTION: is the superiority of risankizumab to ustekinumab sufficient to justify the extra cost?
Let’s see …
My preferred sequencing approach based on the data and factoring in cost will be:
- anti-TNF or ustekinumab biosimilar first-line
- then upadacitinib or risankizumab
We may even think about switching out of class following failure of the first anti-TNF - persistence on the second is never that great even with combination therapy.



I have mild Crohn’s in my ilium. I started with Budesonide which didn’t do much for me. Then after about 8 months on Stelara - fecal cal test, MRI all looked good bye a colonoscopy and capsule test showed I still have mild active Crohn’s. Doctor has me switching to Skyrizi for 6 months to try it before going to remicade/humira. After seeing your sequence I hope that’s not the wrong way to go!