A rush of interesting publications
VIP preprint is live; network meta-analysis in UC; uste vs vedo in TNF failing Crohn's
The last 48 hours has seen a rush of interesting IBD papers come into print:
The main data from the VIP study are available as a preprint
A second network meta-analysis of advanced therapies has been published
Ustekinumab versus vedolizumab after TNF failure in Crohn’s disease
Today’s atomic IBD newsletter outlines these papers, breaking down the main takeaways with commentary.
Reassurance on thiopurines and ustekinumab post-COVID vaccination
The VIP study was set up by Nick Powell at Imperial College London to study the impact of key IBD therapies on response to COVID vaccines.
CLARITY IBD had show us the effect of infliximab compared to vedolizumab, but we were lacking data on ustekinumab, tofacinitib and thiopurines used as monotherapy.
The main data have just appeared in the public domain in pre-print form. These data have not yet completed their journal through formal peer review.
You can access the preprint pdf of the paper here
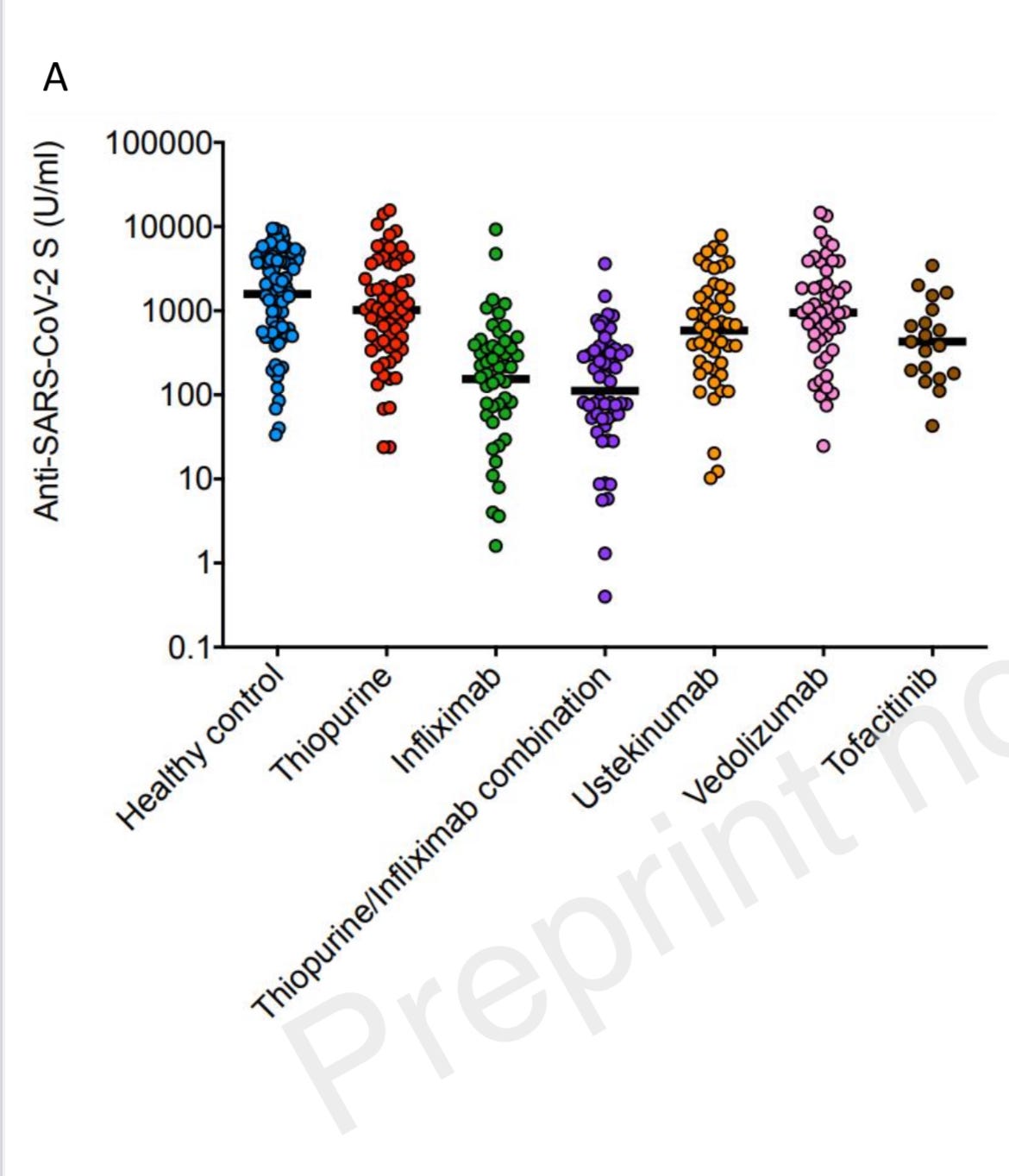
The main figure from the paper shows antibody levels after two doses of vaccine:
Overall, these data should be very reassuring for patients with IBD taking ustekinumab and thiopurines - vaccine responses look normal. The data on tofacitinib suggests some drop in vaccine effectiveness - similar to what we have seen with infliximab. However, these data should be interpreted with a little caution as the sample size was small in the tofacitinib group.
IBD patients who qualify should have had a 3rd primary dose of vaccine. In the UK those who have are eligible for a booster (4th) dose 3 months after dose 3.
Watch the Crohn's Colitis UK Facebook live again here
Network meta-analysis of advanced therapies in UC
We are now truly in the multi-drug era for ulcerative colitis.
Currently available advanced therapies for UC are infliximab (iv and now subcut), adalimumab, vedolizumab, tofacitinib and ustekinumab. A number of additional new molecules have completed their phase 3 clinical trials programmes - filgotinib, etrolizumab, ozanimod and upadacinitib.
There are two methods to compare efficacy and safety profiles of different drugs for an indication:
head to head studies
network meta-analysis
Head to head studies in UC of relevance are: VARSITY (vedolizumab vs adalimumab) and GARDENIA (etrolizumab vs infliximab) and HIBISCUS (etrolizumab vs adalimumab).
Of note, even though the etrolizumab phase 3 programme failed to hit key primary outcomes, the head to head studies provide very useful data to enrich a network meta-analysis (NMA).
We now have two published NMA studies in UC.
Last month Silvio Danese and Laurent Peyrin-Biroulet published the first current systematic review and network meta-analysis (NMA) of advanced therapies for UC in Lancet Gastroenterology and Hepatology.
In this figure you can helpfully see the nodes connecting the studies - most connect back to placebo (placebo controlled RCTs) but some connect directly with each other (head to head studies).
Here is the main display item showing the efficacy results. Their analysis had upadacitinib (JAK1 selective inhibitor) on top across all efficacy analyses. The violet colouring in this figure indicates the comparisons that were statistically significant. There is a lot of violet when you read the comparisons with upadacinitib from left to right.
In terms of safety, vedolizumab came out on top - this is in keeping with expectations and clinical practice. Upadacitinib came out worst when assessing all adverse events but not when assessing serious adverse events (which is a more important safety metric). The conclusion is therefore a bit misleading here I think.
A second NMA for UC published in GUT
Yesterday, Nick Burr and Alex Ford (Alex has published more high quality research than anyone else I know this year) published a second NMA on the same topic in GUT.
The methods for this NMA differ subtly from the LGH paper. There is also a slightly different selection of trials included.
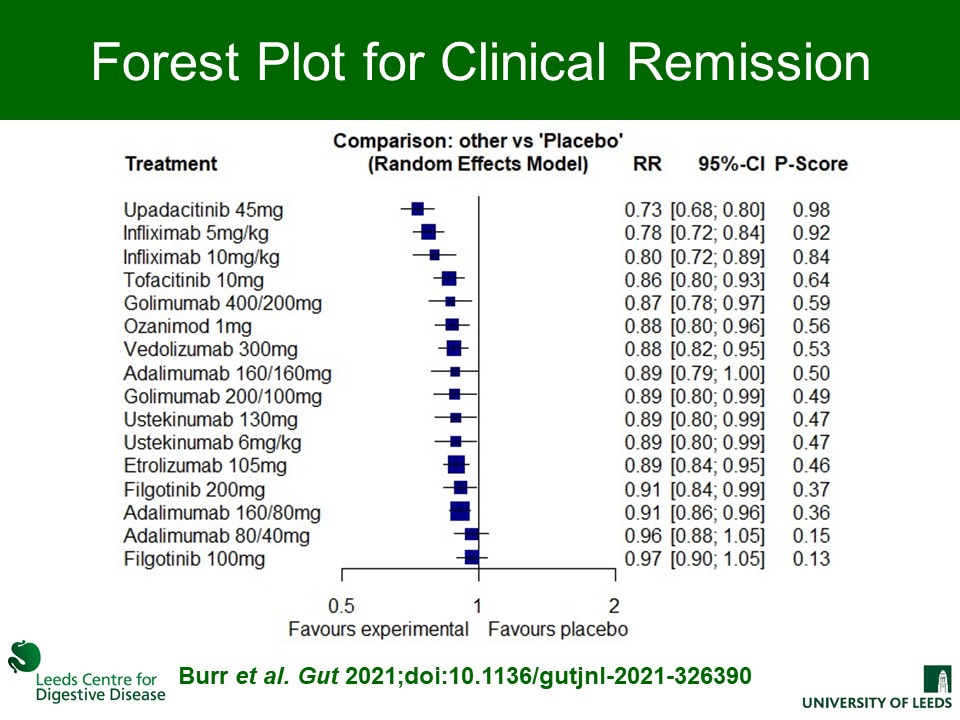
The main results are the same however, with upadacitinib coming out on top for induction of clinical remission. Infliximab was next which is important given the price differential. Infliximab 10mg/kg was most effective endoscopic healing when compared with all other agents except upadacitinib.
Here is the excellent visual abstract from the publication and then a zoomed in view on the main clinical remission forrest plot.
Does this / should this change clinical practice now?
Main messages for me on these two NMAs in UC are:
upadacinitib promises to be a highly effective molecule in the clinic
infliximab remains a very good option in UC
adalimumab performs very poorly in UC
many other molecules show very solid efficacy and safety profiles
Many other important considerations here - notably long-term efficacy and cost.
Adalimumab performs really poorly in all analyses in both studies:
We’ve used very little adalimumab for UC in Edinburgh throughout and even less following VARSITY. However, there is still a lot of adalimuamb first-line prescribing in UC around the UK and globally. There is no denying just how cheap a drug adalimumab now is in biosimilar form.
It may be time to stop using adalimumab in UC.
The NMA data support the on-going use of infliximab in UC:
It is our go to drug in acute severe colitis. Most places now use and accelerated dosing regime in this situation (10mg/kg weekly x 2-3 for induction).
The big problem with infliximab is immunogenicity leading to secondary loss of response. In most circumstances it should be prescribed with an immunomodulator (usually azathioprine) to prevent antibody formation. This does increase the rate of serious adverse events (notably infection) with infliximab).
The advent of subcut infliximab promises to change this IF data support it’s use as a mono therapy agent. Currently this is a big IF, extrapolating from pharmacokinetic profiles. Subcut infliximab is also quite a bit more expensive than iv infliximab in most places.
The NMA data are helpful but should be interpreted with some caution
Head to head studies are the gold standard.
How many more will we see in UC? They are expensive and commercially risky. I would love to see upadacitinib versus infliximab versus vedolizumab with primary outcomes recorded not at 1 year but rather at 2 years.
The long-term durability and safety of vedolizumab is excellent in our clinical experience. The data for ustekinumab is similar although the real-world experience is more lacking in UC. Long-term outcomes are very important for patients.
Ustekinumab versus vedolizumab in TNF failing Crohn’s disease
Anti-TNF therapy utterly dominates first-line position for Crohn’s disease biologics.
However, TNF failure (primary non-response) and secondary loss of response is common, and following TNF other drugs are less effective. This is our experience in the clinic and the data from the RCTs - both vedolizumab and ustekinumab work in Crohn’s disease but the efficacy takes a marked drop when used following TNF.
At least 4 studies have already looked at ustekinumab versus vedolizumab following TNF failure. This week Christian Selinger published a nicely designed multi-centre propensity-matched analysis. Here is the full paper: A propensity score-matched, real-world comparison of ustekinumab vs vedolizumab as a second-line treatment for Crohn’s disease. The Cross Pennine study II
They included 275 patients on ustekinumab and 118 treated with vedolizumab and used propensity matching to deal with any differences in the two groups. This is established methodology which helps to compare different cohorts obtained retrospectively. This is no substitute for a prospective head to head study but nonetheless can provide a useful guide to relative effectiveness and safety.
At week 14 ustekinumab was significantly better than vedolizumab with 38% of the former in clinical remission. However of note, this difference had large gone and was no longer significant at 1 year.
Here you can see a survival analysis of failure rates for both drugs - they are remarkably similar
In practice we typically use ustekinumab in this situation. I think most of the data support that approach.
Both ustekinumab and vedolizumab with be available as biosimilars within 3 years. It would be very interesting to see what happens when you combine both in this setting.
Rizankizumab will be available for Crohn’s disease in 2022. The phase 3 RCT data look very promising in a highly treatment refractory cohort.
Currently we are recruiting to a RCT of rizankizumab versus ustekinumab in this setting. Here are the study details at clinicaltrials.gov
Please register here for IBD Edinburgh 2022